Electronic Effects
Electronic Effects: Overview
This topic covers concepts, such as, Electron Displacement Effects in Covalent Bonds: Electronic Effects, Localised Electrons, Applications of Electromeric Effects & Application of Mesomeric Effect etc.
Important Questions on Electronic Effects
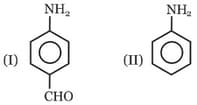
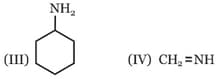
Among these compounds, the correct order of C–N bond length is :
Rank the following free radicals in order of decreasing stability:
In which of the following pairs, is the first species is more stable than second?
In which of the following molecules π-electron density in ring is maximum ?
In which of the following molecules π-electron density in ring is minimum ?
Which of the following compounds has maximum electron density in ring ?
Which of the following compounds has maximum electron density in ring ?
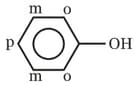
In phenol, π - electron - density is maximum on
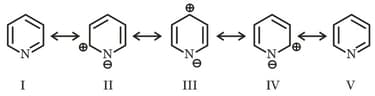
Among these canonical structures of pyridine, the correct order of stability is
The most stable resonating structure of following compound is
For phenol, which of the following resonating structure is the most stable?
In which of the following molecules resonance takes place through out the entire system
The increasing order of basic strengths in their aqueous solutions is:
Hyperconjugation involves overlap of the following orbitals
The reaction of ethene is the presence of can be example for -effect.
Which one of the following does not exhibit electromeric effect?
Ethers do not exhibit electromeric effect but aldehydes and ketones exhibit electromeric effect, why?
Give some examples of the electromeric effect.
Due to which hyperconjugation the dipole moment of alpha-haloalkenes get augmented.
_____ hyperconjugation phenomenon is observed in −halo alkenes.
